A Proposal for TinyTaps
A Digital Health Intervention to Improve Maternity Outcomes and Address Health Inequalities
Abstract
Reduced fetal movement (RFM) is a significant clinical indicator of potential fetal compromise, strongly associated with adverse perinatal outcomes, including stillbirth. Current approaches to fetal movement monitoring face challenges related to inconsistent guidance, user burden, and a lack of integration with NHS clinical pathways. Furthermore, significant health inequalities persist, with women from ethnic minority and socioeconomically deprived backgrounds facing disproportionate risks and additional barriers to accessing timely maternity care. This proposal outlines a multi-phase research programme to address these challenges through the development and evaluation of TinyTaps, a novel digital health intervention. The primary aim is to co-design, implement, and rigorously evaluate an accessible, clinically integrated mobile application that empowers pregnant women to understand their baby's unique movement patterns and facilitates timely communication with their local Maternity Assessment Centre (MAC). The project will follow a phased, mixed-methods approach, beginning with co-design and iterative development (Phase 1) with diverse groups of pregnant women and healthcare professionals at Frimley Health NHS Foundation Trust and Leeds Teaching Hospitals NHS Trust. This will be followed by a pilot feasibility trial (Phase 2) and a comprehensive evaluation using the RE-AIM framework (Phase 3). The study will assess the app's impact on clinical outcomes (appropriate MAC presentations), psychosocial factors (maternal anxiety, fetal attachment), and usability (System Usability Scale). A core focus will be on evaluating the intervention's effectiveness in reducing health inequalities. The project is strategically aligned with the digital transformation and patient-centric priorities of the partner NHS Trusts and national health objectives. Through a robust governance structure, adherence to the highest ethical and data protection standards (HRA, UK GDPR), and a clear pathway to wider implementation, this research aims to deliver an evidence-based tool with the potential to improve maternity safety, enhance patient experience, and promote equitable care across the UK.
1. Introduction
Summary
This section establishes the rationale for the proposed research. It outlines the clinical significance of reduced fetal movements (RFM), identifies the limitations of current practices and technologies, details the health inequalities that exacerbate risks for underserved communities, and demonstrates how this project aligns with the strategic priorities of the NHS and partner Trusts.
Maternal perception of fetal movement is a fundamental indicator of fetal wellbeing, with a significant reduction or sudden alteration in these movements serving as a potentially critical warning sign of impending fetal compromise.[1] The clinical evidence linking RFM to poor perinatal outcomes is substantial and compelling. Systematic reviews and meta-analyses have demonstrated a clear association between RFM and an increased incidence of stillbirth, fetal growth restriction (FGR), and other adverse outcomes.[2, 3] One review found that women presenting with RFM have more than triple the odds of stillbirth (Odds Ratio 3.44) compared to those without such concerns.[3] Furthermore, it is estimated that between 30% and 55% of women who experience an episode of RFM may be at risk of stillbirth within the subsequent week, highlighting the urgency required in clinical assessment.[2, 4]
Despite these well-established links, stillbirth rates in the UK remain a significant public health concern, and recent reviews into maternity care failings have underscored the need for improved systems to prevent avoidable harm.[5, 6] The Royal College of Obstetricians and Gynaecologists (RCOG) Green-top Guideline No. 57, which governs the management of RFM, notes that a majority of women (55%) who experience a stillbirth perceived a reduction in fetal movements prior to the diagnosis.[1] Critically, studies in the UK have identified that an inappropriate or delayed response by clinicians to a mother's report of RFM is a common contributory factor in these tragic outcomes.[1] This evidence establishes that the primary point of failure is often not the absence of a warning sign, but a breakdown in the crucial communication and assessment pathway between the pregnant woman and the healthcare system. The intervention proposed herein is specifically designed to strengthen this pathway by providing a structured, data-rich, and direct channel for communication and triage.
Historically, clinical advice often centred on prescriptive fetal movement counting, such as the "count-to-10" method. However, evidence for the effectiveness of such methods in preventing stillbirth is inconclusive. Cochrane reviews have found insufficient evidence to recommend any single formal counting method over another, or indeed over no formal counting at all.[7, 8] Consequently, contemporary clinical guidance, including that from the RCOG, has shifted away from rigid counting charts towards a more nuanced, individualised approach. The current recommendation is to empower women to become familiar with their baby's unique pattern of movements—including frequency, strength, and timing—and to seek immediate medical advice if they perceive a change from this established norm.[1, 9]
This shift in guidance presents both a challenge and an opportunity. While clinically sound, advising a woman to recognise a "change in pattern" is an abstract concept that can be difficult to self-monitor and can lead to significant anxiety and uncertainty.[10] A simple paper diary is ill-suited to capturing the longitudinal, nuanced data required for pattern recognition. This creates a clear opening for a digital health tool.
The current market of commercial pregnancy applications is extensive and widely used by pregnant women to track various aspects of their pregnancy.[11, 12] However, these apps are fundamentally consumer products, not clinical tools, and suffer from significant limitations. Research has highlighted that many provide scientifically inaccurate information (e.g., misleading fruit-and-vegetable size comparisons for the fetus), have opaque or weak data privacy policies, and are entirely disconnected from clinical care pathways.[11, 12, 13] They do not facilitate communication with NHS services, nor are they designed to align with UK clinical guidelines. The proposed TinyTaps app is designed to fill this specific void. By focusing on pattern recognition, providing simple but effective logging tools, and, most importantly, embedding a direct communication link to the user's registered Maternity Assessment Centre (MAC), it moves beyond a simple consumer product to become an integrated clinical tool.
The burden of adverse perinatal outcomes is not distributed equally across the population. There are marked and persistent inequalities in maternal and neonatal health, with women from ethnic minority backgrounds and those living in the most deprived areas experiencing a significantly higher risk of stillbirth, neonatal death, and other complications.[14, 15, 16] These disparities are compounded by significant barriers that prevent women from these communities from accessing timely and appropriate antenatal care.
Research has identified a range of socio-cultural and systemic factors that inhibit the reporting of RFM. These include a lack of familiarity with the UK healthcare system, language and health literacy barriers, a lack of culturally sensitive care, and a fear of being dismissed or perceived as a burden on the service.[14, 16, 17, 18] Women may delay seeking help due to anxiety about what might be wrong, a fear of unnecessary medical intervention, or a feeling that they will not be taken seriously by healthcare staff.[18]
Mitigating Barriers to Care:
Countering Fear of Dismissal
The app provides an objective, time-stamped log of movements. This transforms the conversation with a midwife from a subjective "worry" to a review of tangible data, empowering the woman and validating her concerns.
Overcoming Language Barriers
The UI is designed to be simple and visual. The co-design phase will prioritise easily understood content, with multi-language integration to reduce reliance on complex English text. Clear instructions will be presented to the user and guidelines for common concerns, misconceptions and things pregnant women may struggle with, e.g. what counts as a movement.[10, 19]
Clarifying the Care Pathway
For women unfamiliar with the NHS, the app removes ambiguity by providing the local MAC's contact number and generating clear alerts, advising the user to seek help and facilitating prompt action. Very simple algorithms can be used to create alerts when the baby's unique patterns have deviated, removing responsibility and burden from the mother, also giving her peace of mind when baby's movements are normal.[10, 19]
Accessible and Easy-to-Use
The app can leverage the wide range of accessibility features available on modern mobile phones, allowing for easy logging of movements without the burden often found in many other apps.
The proposed research programme is designed to align directly with the strategic objectives of the initial partner organisations: Frimley Health NHS Foundation Trust and Leeds Teaching Hospitals NHS Trust.
Frimley Health NHS Foundation Trust (FHFT)
FHFT's 2030 strategy emphasizes modernising infrastructure, leveraging digital tools, and becoming a "leader in digital".[20, 21, 22] The Trust plans to use remote monitoring and virtual wards to empower patients and has a stated objective to address health inequalities.[21, 22, 23] This project is a direct fit, offering a modern, digital, patient-centric intervention that addresses a key clinical need and aligns with the Trust's commitment to innovation and health equity.[24]
Leeds Teaching Hospitals NHS Trust (LTHT)
LTHT is a major teaching hospital with a world-leading research culture and an "Innovation Pop Up" to translate new technologies into solutions.[25, 26, 27, 28] The Trust's Digital IT Strategy aims to "lead on innovation and research" and form partnerships with academia and industry.[29] Recent CQC inspections (20 June 2025) rated the Trust's maternity services as 'inadequate', highlighting concerns with safety and risk management.[30, 31] This project presents a timely opportunity for LTHT with an innovative, evidence-based digital solution for maternity safety. Even though points of concern were "learning following incidents, risk management, safe environment, infection prevention and control, medicines management and some governance processes", services like "TinyTaps" can help to improve the culture to aid in addressing some of the more serious issues in the future. We also find Leeds to be well-suited as a city to pilot this research - the UK's third largest city, extremely culturally and religiously rich and diverse, and recently reaffirmed as the best place in the UK for health technologies, following the meeting with the Secretary of State and CEO of the new Medicines and Healthcare products Regulatory Agency hub.
2. The Proposed Solution: TinyTaps
Summary
TinyTaps is a secure, NHS-integrated mobile app to help pregnant women learn their baby's unique movement patterns. It provides reassurance and, if a significant change is detected, gently alerts the mother with clear instructions to contact her local Maternity Assessment Centre (MAC). The primary user is the pregnant woman; secondary users are midwives who can use the app's data to inform clinical assessments. This section outlines the project's aims, objectives, and core hypotheses, focusing on usability, clinical effectiveness, and health equity.
TinyTaps is a secure, easy-to-use mobile application for pregnant women, designed to be recommended by their midwife. It helps women learn the unique pattern of their baby's movements and provides reassurance. If the app detects a significant change in the pattern, it will gently alert the mother and provide clear, simple instructions on how to contact her local NHS Maternity Assessment Centre (MAC).
The primary user is the pregnant woman, who will use the app daily from around 18-24 weeks of gestation until birth. The value for her is peace of mind, a reduced sense of burden, and the confidence that she is being supported by an NHS-integrated tool. The effort required is minimal: a few simple taps on her phone to log movement sessions.
Secondary users are the midwives and clinicians at the MAC. When a woman presents with concerns, the midwife can review the objective, time-stamped data from the app, leading to a more informed clinical assessment. The app is intended to interface with NHS patient record systems in the long term, allowing movement data to become part of the woman's official maternity record.
Primary Aim
To co-design, implement, and evaluate a clinically integrated, accessible mHealth application (TinyTaps) that empowers pregnant women, particularly those from underserved communities, to understand their baby's unique movement patterns and to seek timely and appropriate clinical assessment for RFM.
Key Objectives
Co-design & Usability
To co-design and develop a user-centred prototype with diverse pregnant women, midwives, and obstetricians.[33, 34]
Clinical Integration
To pilot a clear and feasible clinical pathway for the app's integration into routine antenatal care.[24, 25, 35]
Clinical Effectiveness
To assess the app's effect on the frequency and appropriateness of presentations to MACs for RFM.[2, 4]
H1: Increased Appropriate Presentations
The use of TinyTaps will lead to a statistically significant increase in appropriate presentations to Maternity Assessment Centres for reduced fetal movements.
H2: High Usability
The app will achieve a mean System Usability Scale (SUS) score of >70, indicating "good" to "excellent" usability.[39, 40]
H3: No Increase in Anxiety
The app will not lead to a statistically significant increase in maternal anxiety, as measured by the General Anxiety Disorder-7 (GAD-7) scale.
H4: Equitable Usability
There will be no statistically significant difference in mean SUS scores between women from White British backgrounds and those from ethnic minority backgrounds.
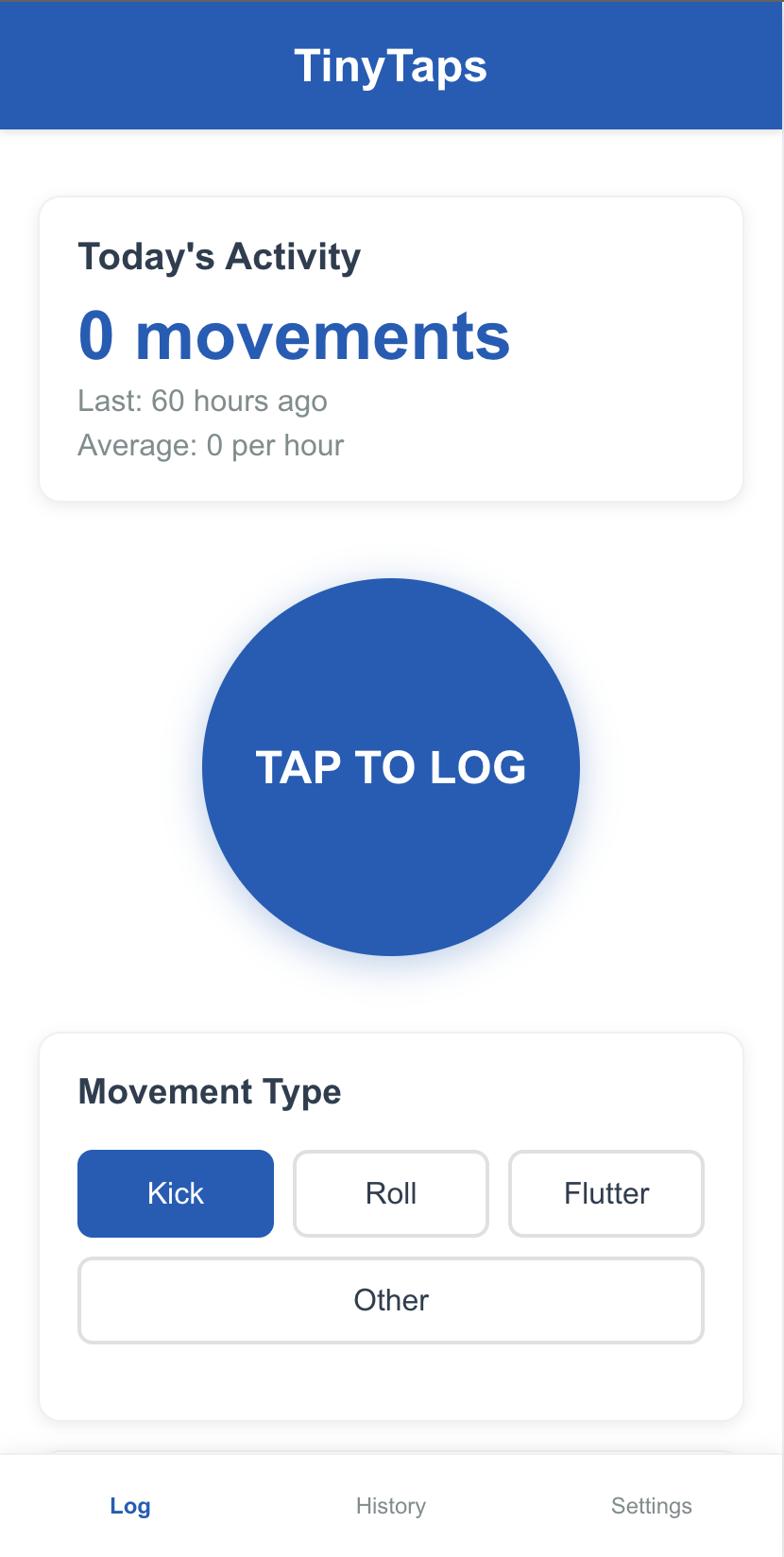
Log Movement (1)
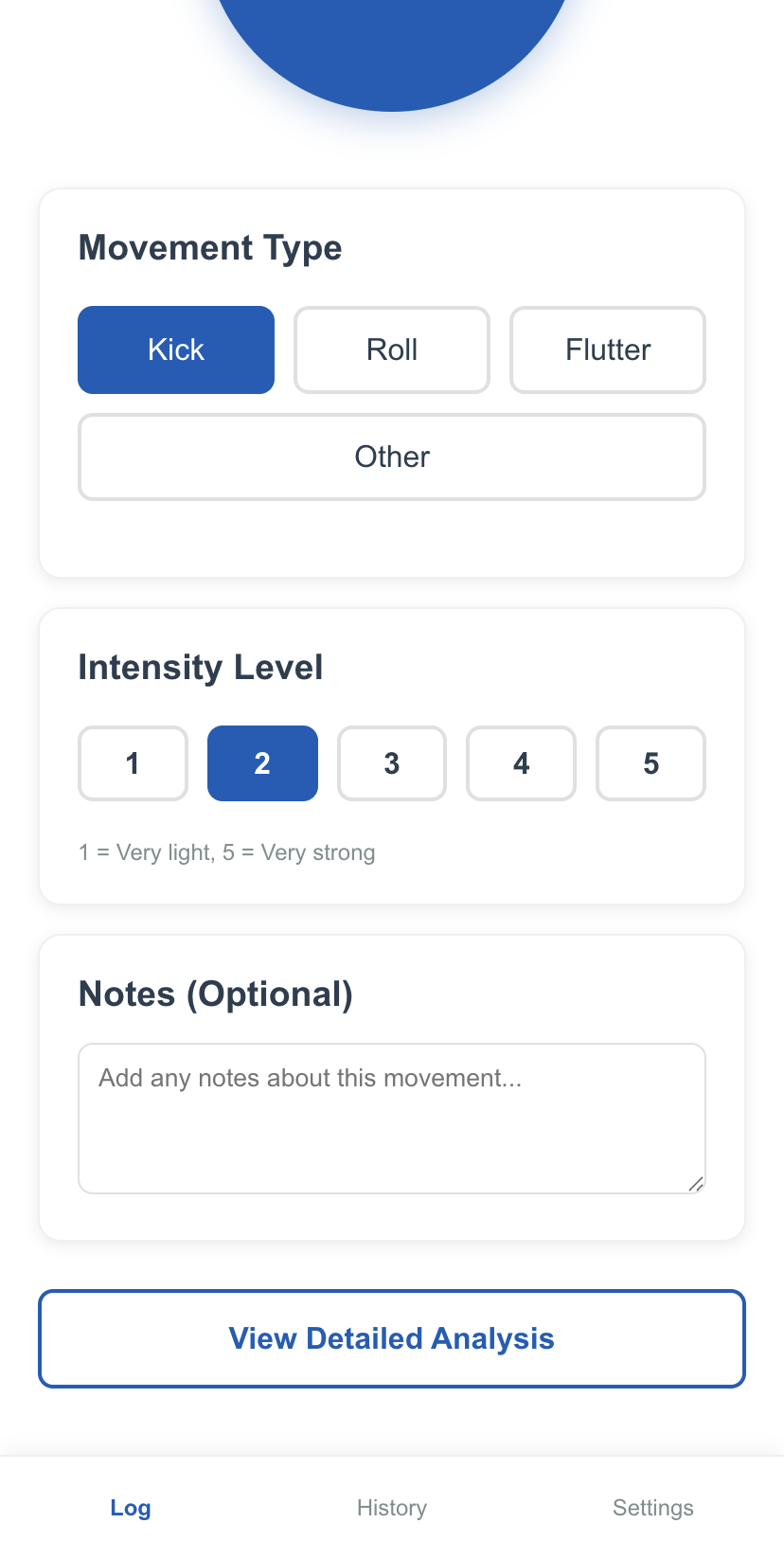
Log Movement (2)
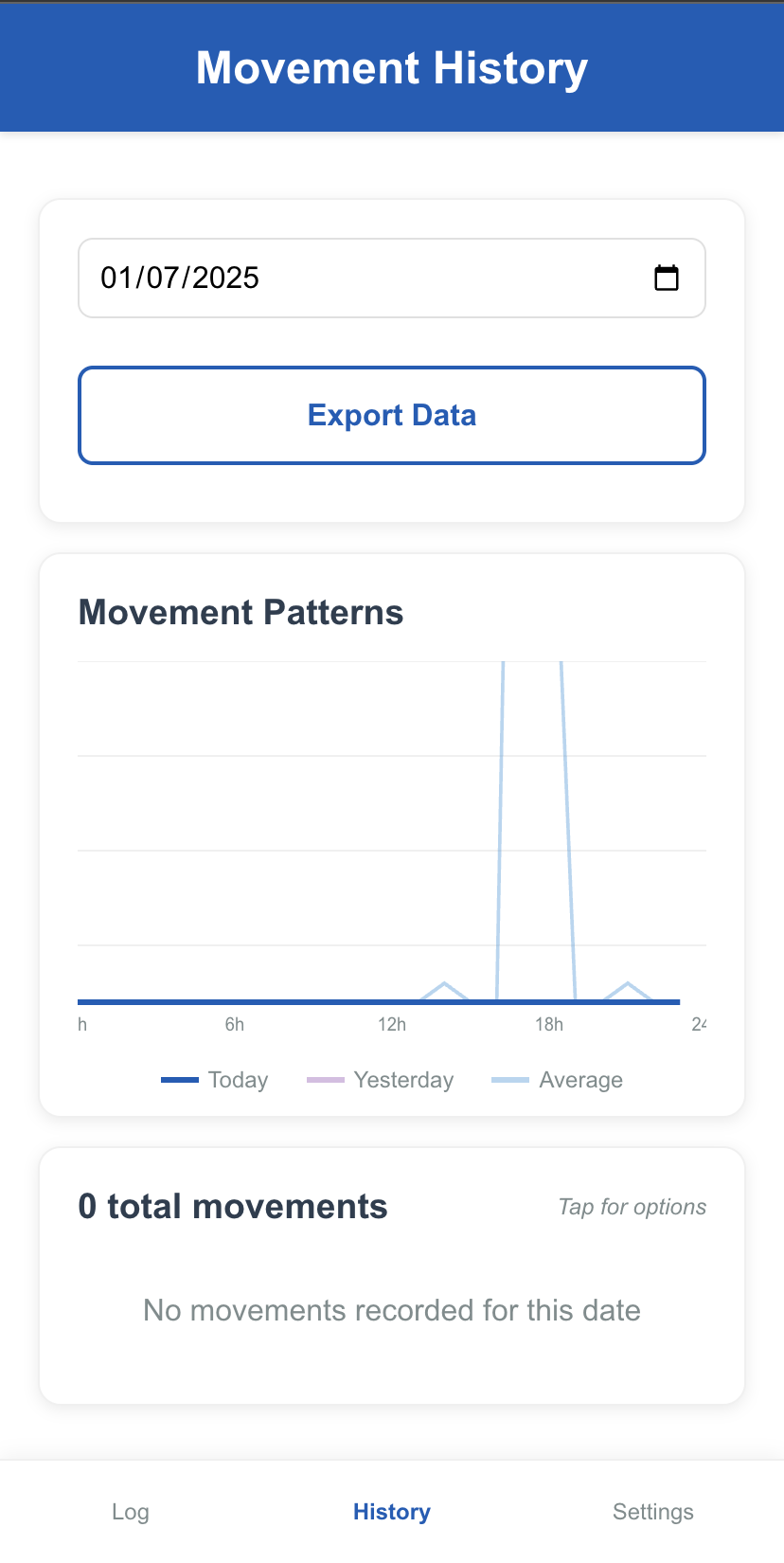
History & Patterns
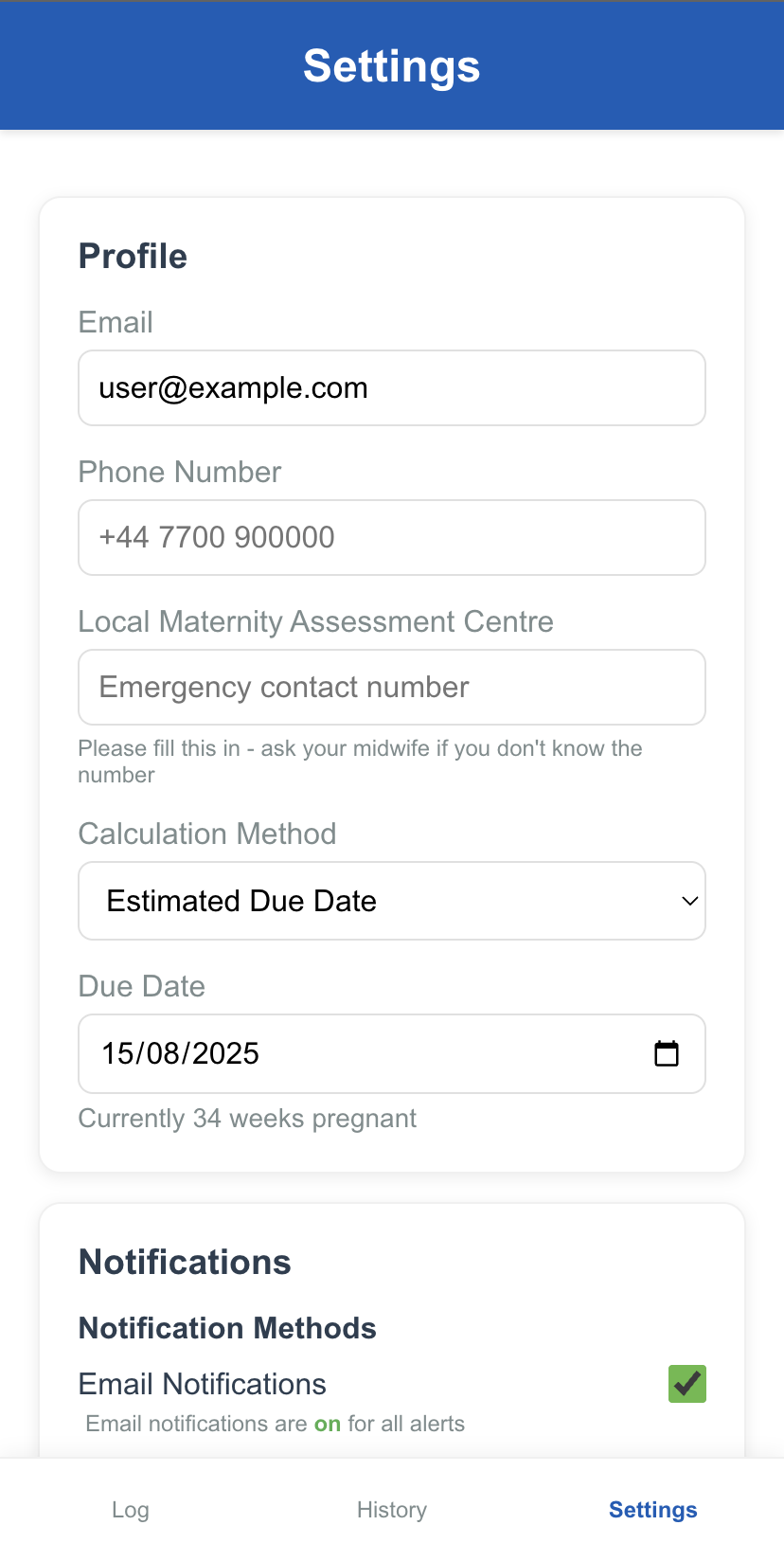
Profile & Notifications
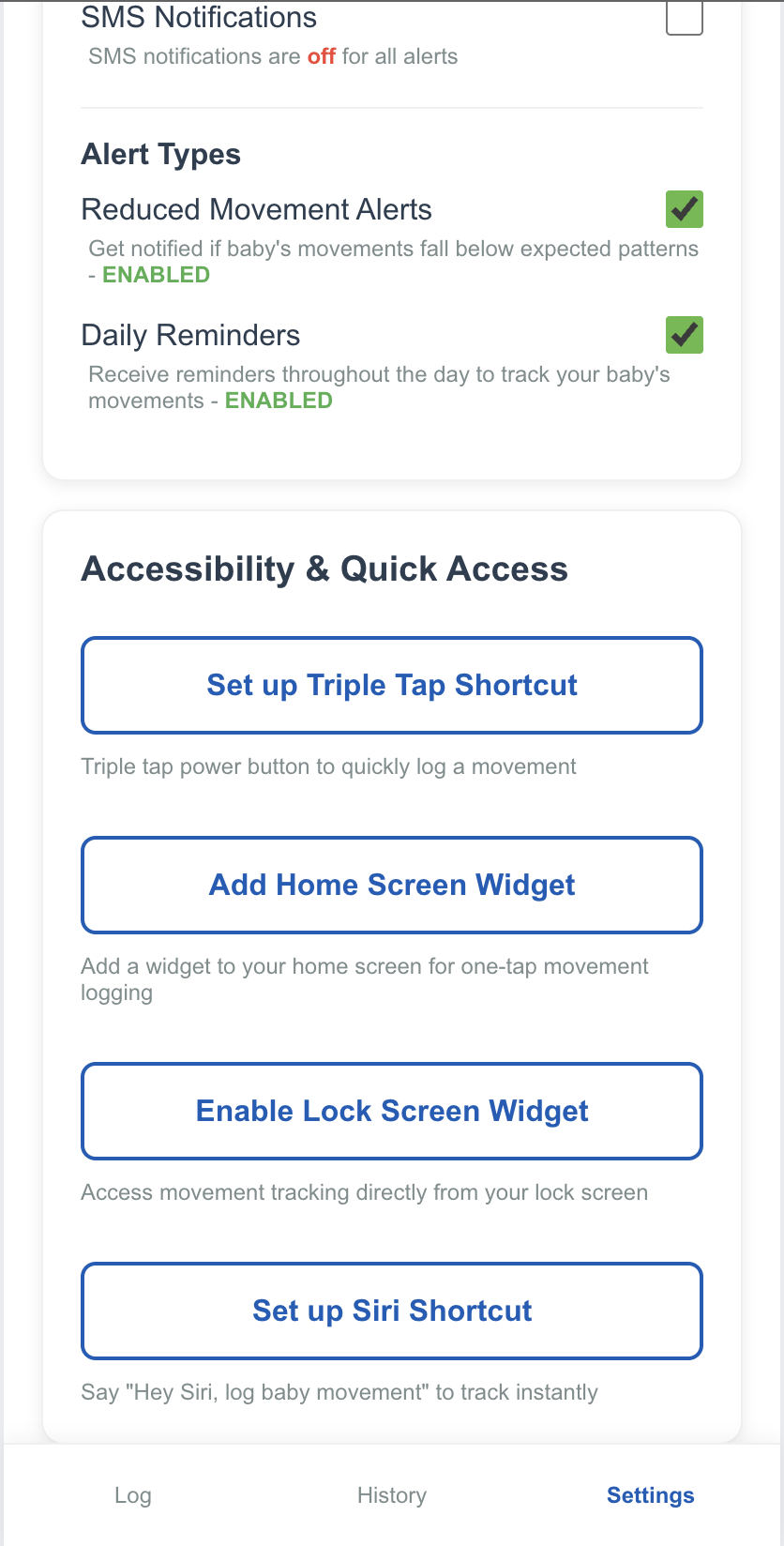
Accessibility & Shortcuts
- Pregnant Women: The primary users. Their interest is in a simple, reassuring, and effective tool to monitor their baby's health, reduce anxiety, and feel empowered in their communication with healthcare providers.
- Midwives and Obstetricians: Key clinical users. Their interest is in a tool that provides objective, reliable data to support clinical decision-making, improves the efficiency and appropriateness of MAC presentations, and helps them provide better, more equitable care.
- NHS Trusts (Frimley Health FT, Leeds Teaching Hospitals NHS Trust): The implementing organisations. Their interest lies in improving maternity safety metrics, reducing stillbirth rates, addressing health inequalities, and advancing their digital transformation strategies in a cost-effective manner.
- Researchers and Academics: Their interest is in generating high-quality evidence for the effectiveness of digital health interventions, contributing to the academic literature, and informing national policy and clinical guidelines.
3. System Requirements and Design
Summary
This section details the technical blueprint for TinyTaps. It outlines functional requirements through a use case diagram and specifies key non-functional requirements such as security, usability, and interoperability. A user story illustrates the app's practical application, and a deployment diagram shows the secure, scalable client-server architecture built on modern cloud technologies (React Native, Node.js/Python, secure database) designed for future integration with NHS systems via FHIR APIs.
The use case diagram below illustrates the primary users and their interactions with the TinyTaps system. The system boundary encapsulates all core functionality.
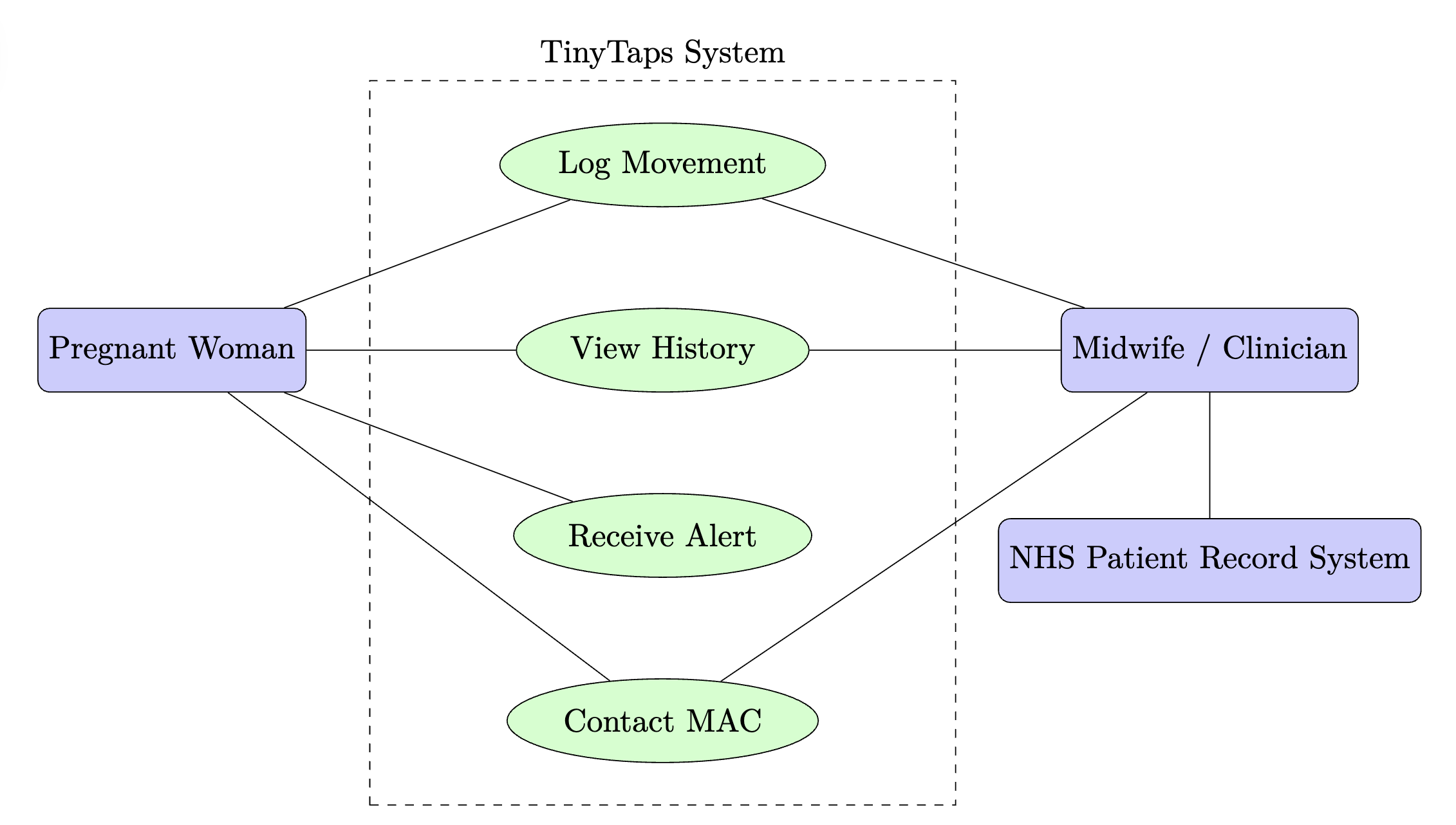
Use Case Narrative
A Pregnant Woman is the primary actor. Her value proposition is gaining reassurance and a clear pathway to care. She interacts with the system to Log Movement, View History to understand her baby's patterns, and Receive Alerts if a potential issue is detected. The app provides a direct function to Contact MAC. A Midwife/Clinician is a secondary user. Their value is access to objective data for better clinical assessment. They can Review Patient Data presented by the woman. In future iterations, the system will interface with the NHS Patient Record System (a system actor) to automatically update a woman's record.
- Security: Must comply with UK GDPR and NHS data security standards (DTAC). All data must be encrypted in transit and at rest.
- Usability & Accessibility: The app must be intuitive with minimal user effort. It must achieve a SUS score >70 and comply with WCAG 2.1 AA accessibility standards.
- Reliability: The system must have >99.9% uptime. The alerting algorithm must be robust and fail-safe.
- Performance: UI response time must be <2 seconds for all user interactions. Data processing for pattern analysis must be completed in near real-time.
- Interoperability: The system must be designed with future integration with NHS systems (e.g., via FHIR APIs) in mind.
- Maintainability: The codebase must be well-documented and modular to allow for straightforward updates and feature additions.
User Story: Aisha is a 30-year-old solicitor, 32 weeks into her first pregnancy. Her midwife at Frimley Park Hospital recommended she download TinyTaps.
One evening, while resting after work, Aisha feels her baby has been quieter than usual. She opens the TinyTaps app. Instead of trying to remember and count, she simply starts a "Movement Session". Over the next hour, she taps a large, simple button on the screen each time she feels a movement. The app requires minimal interaction, so she can continue relaxing.
After the session, she looks at the "History" screen. She can see a graph of her baby's movements over the past few weeks. The app's simple algorithm highlights that today's activity is significantly below the baby's established baseline. A calm, non-alarming notification appears: "Your baby's movements seem a little quieter than usual today. It's always best to get checked out. Please call your Maternity Assessment Centre for advice." The app displays the direct "Mama's Line" phone number for Frimley Park's MAC.
Aisha calls the number. The midwife asks her to come in. At the hospital, Aisha shows the midwife her movement history on the app. The midwife is able to see the clear, time-stamped data, which validates Aisha's concerns and provides an objective basis for the clinical assessment. This empowers Aisha and facilitates a more effective and efficient consultation.
Implementation Diagram
The proposed solution uses a modern, secure, and scalable client-server architecture, as shown in the deployment diagram.
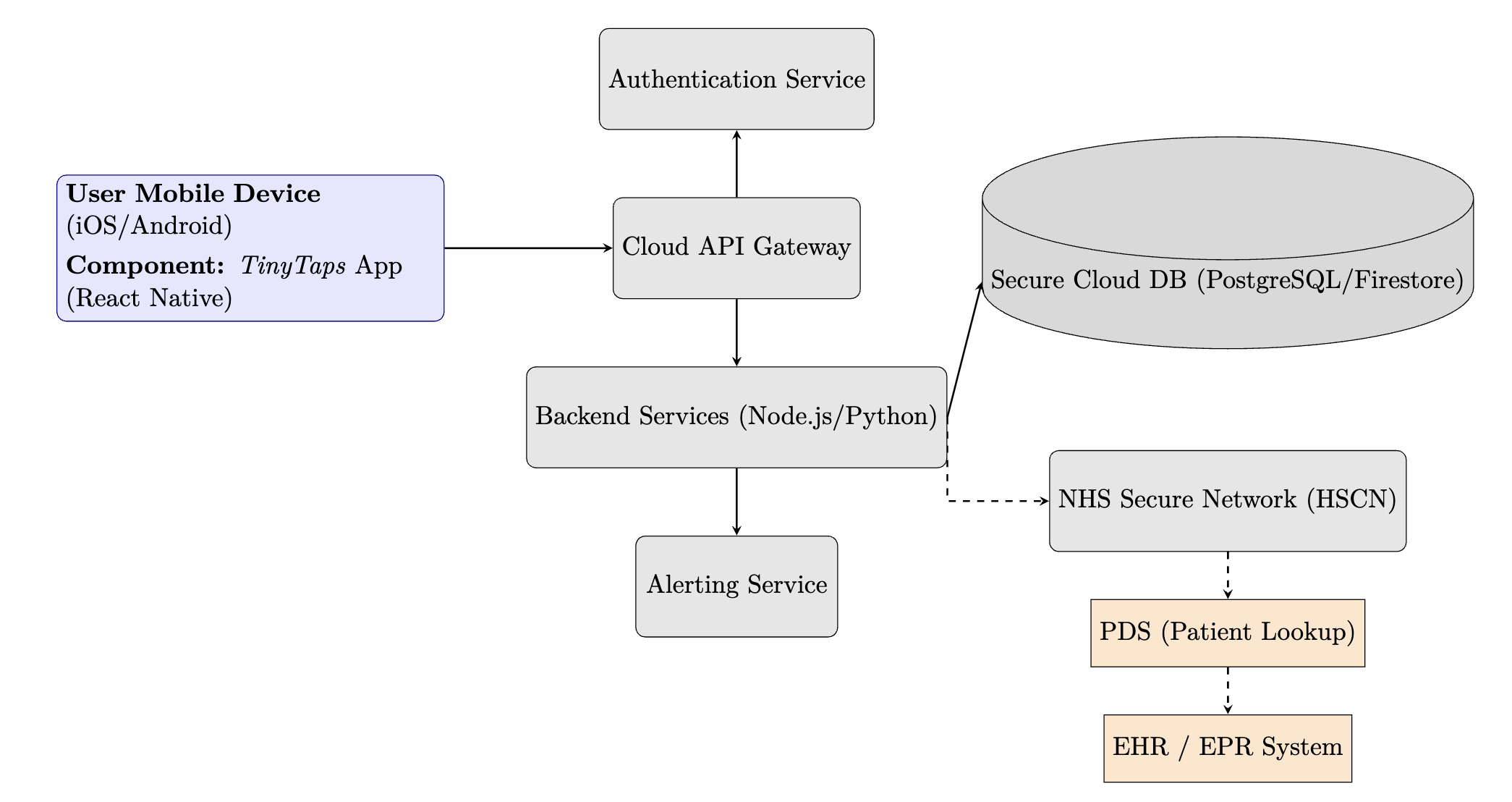
Technology and Infrastructure
- Hardware/Networking: No new hardware is required for end-users, who will use their own smartphones. The backend infrastructure will be hosted on a secure, UK-based cloud platform (e.g., AWS London Region, Microsoft Azure UK South) that is compliant with NHS standards. Connectivity to the NHS Health and Social Care Network (HSCN) will be required for future EHR integration.
- Software and Platforms:
- Mobile App: Developed using React Native for cross-platform compatibility (iOS and Android) from a single codebase.
- Backend: A microservices architecture using Node.js or Python, deployed in Docker containers for scalability.
- Database: A secure, managed SQL (e.g., PostgreSQL) or NoSQL (e.g., Firestore) database with encryption at rest and regular backups.
- Interfaces: A secure RESTful API will be used for communication between the mobile app and the backend. Future interfaces to NHS systems will use the FHIR standard. Although not necessary for the app to function, this would enable clinicians to access patient data directly, integrated within patient records, as well as patient login authentication through their NHS account.
- Addressing Non-Functional Requirements:
- Security will be addressed through end-to-end encryption, secure authentication (e.g., OAuth 2.0), regular penetration testing, and adherence to the NHS DTAC.
- Reliability and Performance will be managed through cloud-native tools, including load balancing, auto-scaling, and performance monitoring services (e.g., AWS CloudWatch).
4. Study Framework
Summary
This study adopts a three-phase, mixed-methods design based on the MRC framework. Phase 1 (Months 1-9) is dedicated to co-design and iterative development with diverse users. Phase 2 (Months 10-24) involves a pilot feasibility trial to test the intervention and protocol. Phase 3 (Months 25-36) is a comprehensive evaluation using the RE-AIM framework to assess real-world Reach, Effectiveness, Adoption, Implementation, and Maintenance.
This study will adopt a phased, mixed-methods design, consistent with the Medical Research Council (MRC) framework for developing and evaluating complex interventions and the NHS HEE Research Toolkit.[41, 42] The programme is divided into three sequential phases.
The successful adoption and effectiveness of digital health interventions (DHIs) are critically dependent on meaningful end-user involvement from the earliest stages of development.[33] A co-design methodology, which treats end-users as active partners in the design process rather than passive recipients of technology, has been shown to produce more engaging, relevant, and effective solutions.[34, 43] This phase will therefore employ a human-centred design approach to refine the initial app prototypes into a high-fidelity, clinically robust tool.
Methodology:
-
Recruitment: A purposive sampling strategy
will be employed to recruit participants for three distinct
co-design groups at both the Frimley and Leeds sites.
- Pregnant & Post-partum Women (n=~20 per site): The sample will be stratified to ensure diversity in terms of ethnicity, socioeconomic status, parity, and self-reported digital literacy.[14, 16]
- Midwives (n=~10 per site): A mix of community, hospital-based, and specialist midwives will be recruited.
- Clinical Staff (n=5 per site): Obstetricians and senior staff from the MACs will be recruited.
- Co-Design Activities: [33] A series of iterative activities will be conducted, including Focus Groups, Workshops, and Usability Testing, to refine the UI/UX, content, and features.
Following the MRC framework, a pilot study is essential to test the feasibility of the intervention and the research protocol before committing to a large-scale trial.[42]
Methodology:
A two-arm, non-randomised, controlled feasibility trial will be conducted.
- Participants: 100 pregnant women at >18 weeks of gestation will be recruited at each site (50 intervention, 50 control), all recruited at either the first (dating) or second (anomaly) routine scans.
- Intervention Group: Participants will be introduced to the TinyTaps app by their midwife.
- Control Group: Participants will receive standard antenatal care.
- Data Collection: A mixed-methods approach will be used[44], collecting quantitative data (demographics, app analytics, MAC presentations, SUS (System Usability Scale)[39, 45], GAD-7 (General Anxiety Disorder-7)[37, 38]) and qualitative data (interviews with participants and focus groups with healthcare professionals).
The evaluation will be structured using the RE-AIM QuEST framework, a robust model to assess real-world implementation and public health impact.[46]
Table 1: RE-AIM Evaluation Framework for TinyTaps [46, 47, 48]
| Dimension | Research Question(s) | Data Source(s) | Analysis Method |
|---|---|---|---|
| Reach | Who is using the app? Does it reach underserved groups? | App download and registration data; Baseline demographics; Clinic statistics. | Descriptive statistics. Chi-square tests to compare user demographics against clinic population. |
| Effectiveness | What is the impact on clinical and psychosocial outcomes? | MAC attendance logs; CTG records; Validated questionnaires (GAD-7, SUS); Qualitative interviews. |
Quantitative: Inferential statistics
(e.g., logistic regression, t-tests).
Qualitative: Thematic analysis of interview transcripts. |
| Adoption | Are midwives, clinicians, and organisations willing to adopt the intervention? | Stakeholder interviews; Clinic-level uptake rates. | Thematic analysis of interview data to identify organisational barriers and facilitators. |
| Implementation | Is the intervention being delivered and used as intended? | App usage analytics; Audio-recordings of midwife consultations; Interviews with midwives. | Analysis of app usage patterns. Fidelity checklist analysis. Thematic analysis of interviews. |
| Maintenance | What is required to sustain the intervention long-term? What is the cost-effectiveness? | Follow-up surveys; Interviews with Trust leads and commissioners; Health economic analysis. | Longitudinal data analysis. Thematic analysis of manager interviews. Cost-utility analysis. |
5. Governance, Ethics, and Data Integrity
Summary
The project will adhere to the highest regulatory standards. As a Software as a Medical Device (SaMD), the app requires MHRA and HRA/REC approval via the IRAS system. A robust Patient and Public Involvement and Engagement (PPIE) strategy is central to the project. Full compliance with UK GDPR is ensured through a documented lawful basis for processing sensitive health data, explicit user consent, a Data Protection Impact Assessment (DPIA), and a comprehensive Data Management Plan (DMP).
This project will be conducted to the highest standards of research governance, ethical practice, and data protection, in full compliance with UK regulatory frameworks.
The TinyTaps app qualifies as Software as a Medical Device (SaMD).[49, 50] The project requires approval from the MHRA and a HRA-managed REC.[49, 51] The application will be submitted via the Integrated Research Application System (IRAS) using the combined review service.[52, 53, 54] We will work closely with the R&D offices at both partner Trusts to gain HRA and HCRW Approval and confirm capacity and capability.[24, 55, 56, 57, 58]
Meaningful PPIE is a core component of this research, adhering to NIHR best practice.[59, 60, 61] Our strategy includes recruiting pregnant and post-partum women as co-designers (compensated in line with NIHR guidance[61, 62]) and establishing a diverse PPIE advisory group to review participant-facing materials and co-develop the dissemination strategy.[5] We will partner with community organisations to ensure inclusivity.[14, 16, 63]
Processing of special category data will comply with UK GDPR and the Data Protection Act 2018.[64, 65, 66] Measures include:
- Lawful Basis: Documented reliance on Article 6(1)(e) 'Public Task' and Article 9(2)(j) 'Research purposes' and/or 9(2)(h) 'Health or social care'.[67, 68, 69]
- Explicit Consent: A clear, granular, and unbundled consent process.[67, 70, 71]
- DPIA: A comprehensive Data Protection Impact Assessment will be conducted.[65, 72]
- Transparency: An accessible, simple-to-digest and understandable Privacy Policy detailing user rights.[73, 74]
- Security: Data minimisation, pseudonymisation, and robust encryption measures.[64, 75]
6. Project Management, Timeline, and Dissemination
Summary
A multidisciplinary team will manage the project, overseen by a steering committee with partner Trust and PPIE representation. A 36-month timeline is proposed, beginning with rapid app deployment and data collection to inform a large-scale pilot controlled trial. Findings will be disseminated widely to academic, NHS, public, and policy audiences to maximize impact and support UK-wide adoption.
This section details the operational plan for the project, including the team structure, a detailed timeline with key milestones, and a comprehensive strategy for disseminating the research findings to ensure maximum impact.
A multidisciplinary team will be assembled (PI, Clinical Co-Is, Project Manager, Research Midwives, Software Lead, Statistician). A Project Steering Committee with strategic oversight will meet quarterly, comprising the core team, Trust R&D representatives, a PPIE lead, and an independent chair.
The project is planned over a 36-month period. An accelerated initial phase will see the app developed and deployed within 3 months to enable immediate observational data collection, informing the main controlled trial. This phased approach is a crucial component of modern funding applications.[79, 80]
Table 2: Project Timeline (Gantt Chart)
| Phase | Work Package | M1-3 | M4-6 | M7-9 | M10-12 | M13-18 | M19-24 | M25-30 | M31-36 |
|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | 1.1 App Finalisation & App Store Submission | ||||||||
| 1.2 IRAS/HRA/MHRA Application Submission | |||||||||
| 1.3 Initial Co-Design & Usability Testing | |||||||||
| Milestone 1: App Live & Ethics Submitted | ✓ |
||||||||
| Phase 2 | 2.1 Ethical Approval & Site Setup | ||||||||
| 2.2 Real-world Data Collection (Observational) | |||||||||
| 2.3 Ongoing Co-design & App Iteration | |||||||||
| Milestone 2: Pilot Trial Protocol Finalised | ✓ |
||||||||
| Phase 3 | 3.1 Pilot Trial Recruitment & Data Collection | ||||||||
| 3.2 Qualitative Data Collection (Interviews) | |||||||||
| 3.3 Data Analysis & Manuscript Preparation | |||||||||
| Milestone 3: Final Report & Grant Submission | ✓ |
||||||||
A multi-channel dissemination strategy will be implemented to ensure findings reach all relevant audiences.[25, 47, 81]
- Academic Audiences: Publication in high-impact, peer-reviewed journals and presentations at key conferences.[25]
- NHS Stakeholders: Bespoke reports for Trust Boards, R&I departments, and sharing with regional Health Innovation Networks.[22]
- Public and Participants: Co-created accessible outputs (lay summaries, infographics) disseminated via partner and charity websites.[6, 82]
- Policy and Implementation Impact: The evidence will form a business case for wider NHS commissioning and inform national guidance.
7. Resource Requirements and Funding Strategy
Summary
The project requires funding for staffing, app development, participant costs, and dissemination. A phased funding strategy will be employed, initially seeking smaller grants (e.g., NIHR i4i Connect, MRC DPFS 'Gap Fund') for co-design and feasibility work, followed by a larger application (e.g., NIHR PGfAR) to support the full-scale evaluation and implementation.
This section outlines the required financial resources and a phased strategy for securing funding.
A phased funding strategy allows for the generation of pilot data to de-risk the project for a larger programme grant application. This aligns with the translational funding pathways of the NIHR and MRC.[84, 85, 86]
Table 3: Phased Funding Strategy
| Phase | Target Funding Stream(s) | Amount | Key Activities Funded |
|---|---|---|---|
| Phase 1: Co-Design & Prototype Validation (M1-12) | NIHR Invention for Innovation (i4i) Connect [85, 87] or MRC DPFS "Gap Fund" [84] | £100k–£150k | Co-design, prototype development, usability testing, ethics application. |
| Phase 2 & 3: Pilot & Full Evaluation (cRCT) (M13-36+) | NIHR Programme Grant for Applied Research (PGfAR) [47, 88] or NIHR i4i Product Development Award (PDA) [85, 89] | £2M–£3.5M | Full multi-site cRCT, RE-AIM evaluation, health economic analysis, pathway to UKCA marking. |
| Cross-cutting Theme | NIHR Challenge: Maternity Inequalities [63] or Wellcome Trust Mental Health Awards [90, 91] | (Supplements) | Work packages on health disparities and perinatal anxiety. |
8. Conclusion
Summary
This proposal details a methodologically sound and achievable plan to develop and evaluate TinyTaps. The project is grounded in robust software engineering and research principles, with a phased timeline and funding strategy to ensure feasibility. While acknowledging limitations like user dependency and EHR integration complexities, the proposal presents a clear pathway to creating an evidence-based tool that empowers women, supports clinicians, and has the potential to save babies' lives.
This proposal outlines a robust, multi-phase programme to develop and evaluate TinyTaps, a digital health intervention with the potential to significantly improve maternity safety and address health inequalities.
Sound Software Engineering Principles
The project is founded on sound software engineering principles. It begins with a thorough investigation of user and system requirements, incorporates a user-centred, co-design methodology, and follows an iterative development lifecycle. The proposed architecture is scalable, secure, and maintainable, and explicitly addresses key non-functional requirements. By separating the research methodology from the system design, we ensure both clinical and technical rigour.
Achievability
The phased timeline and budget strategy make this ambitious project achievable. By securing initial funding for the co-design and feasibility phases, we de-risk the project and build a strong evidence base to justify the larger investment required for the full-scale trial. The project plan includes clear milestones to monitor progress.
Limitations and Outstanding Issues
The primary limitation is that TinyTaps is an assistive tool, not a diagnostic one; its effectiveness is dependent on user engagement and correct use. The accuracy of the pattern-detection algorithm is another challenge that will require careful validation during the pilot phase. Outstanding issues to be addressed in the long term include the complexities of full, bi-directional integration with the diverse landscape of NHS Electronic Health Record systems and establishing a sustainable long-term funding model for the app's maintenance and support post-study.
Despite these challenges, this proposal presents a clear and achievable pathway to developing an evidence-based tool that can empower women, support clinicians, and ultimately save babies' lives.
References
- Royal College of Obstetricians and Gynaecologists. (2011). Reduced Fetal Movements (Green-top Guideline No. 57). RCOG.
- Frøen, J. F., Heazell, A. E., Tveit, J. V., Saastad, E., Fretts, R. C., & de-Bernis, L. (2011). Fetal movement counting for sensing fetal wellbeing. Cochrane Database of Systematic Reviews, (3).
- Mangesi, L., & Hofmeyr, G. J. (2007). Fetal movement counting for assessment of fetal wellbeing. Cochrane Database of Systematic Reviews, (1).
- O'Sullivan, O., Stephen, G., Martindale, E., & Heazell, A. E. (2009). An estimation of fetal-maternal haemorrhage post-delivery: a prospective study. BJOG: An International Journal of Obstetrics & Gynaecology, 116(3), 438-444.
- Ockenden, D. (2022). Final report of the Ockenden review. Independent review of maternity services at Shrewsbury and Telford Hospital NHS Trust.
- Sands. (n.d.). Saving Babies' Lives.
- Heazell, A. E., & Froen, J. F. (2008). Methods of fetal movement counting and the detection of fetal compromise. Journal of Obstetrics and Gynaecology, 28(2), 147-154.
- Bellussi, F., Po, G., Livi, A., et al. (2020). Fetal movement counting and stillbirth prevention: a systematic review and meta-analysis of randomized controlled trials. American Journal of Obstetrics and Gynecology, 223(4), 509-519.e2.
- Tommy's. (n.d.). Feeling your baby move: a guide for mums-to-be.
- Daly, L. M., Horey, D., Middleton, P. F., & Flenady, V. (2021). Women’s and clinicians’ experiences, views and preferences for fetal movement counting: a mixed-methods systematic review. BJOG, 128(1), 16-29.
- Thomas, G. M., & Lupton, D. (2016). ‘Precious, precious, precious’: the agential intra-actions of digital technologies in the context of pregnancy. Qualitative Health Research, 26(1), 77-87.
- Hughson, J. P., Daly, J. O., Woodward-Kron, R., et al. (2018). The rise of pregnancy and parenting apps and the implications for clinical practice. Medical Journal of Australia, 209(9), 385-387.
- Lupton, D., & Thomas, G. M. (2015). ‘Playing life’s biggest game’: pregnancy apps and the gamification of prenatal care. Health Sociology Review, 24(3), 256-269.
- MBRACE-UK. (2021). Perinatal Mortality Surveillance Report - UK Perinatal Deaths for Births from January to December 2019. The Infant Mortality and Morbidity Studies, University of Leicester.
- Knight, M., Bunch, K., Tuffnell, D., et al. (Eds.). (2021). Saving Lives, Improving Mothers’ Care 2017-19. National Perinatal Epidemiology Unit, University of Oxford.
- NHS Race and Health Observatory. (2022). Ethnic Inequalities in Healthcare: A Vicious Cycle.
- Kingdon, C., Downe, S., & Betts, D. (2012). The role of cultural context in the experience of and response to reduced fetal movements in a multi-ethnic population. Women and Birth, 25(2), 65-72.
- Heazell, A. E. P., & Green, J. M. (2016). Women's experiences of and attitudes to the management of reduced fetal movements in a UK hospital. Midwifery, 37, 52-58.
- Kennelly, M. A., Ainle, F. N., D'Arcy, R., et al. (2017). The Maternal Health Literacy Project (MHLP): A cohort study protocol. BMC Pregnancy and Childbirth, 17(1), 1-8.
- Frimley Health NHS Foundation Trust. (2023). Our 2030 Strategy.
- Frimley Health NHS Foundation Trust. (2022). Annual Report and Accounts 2021/22.
- Frimley Health and Care ICS. (n.d.). Digital Strategy.
- Frimley Health NHS Foundation Trust. (2021). Equality, Diversity and Inclusion Annual Report 2020-21.
- Frimley Health NHS Foundation Trust. (n.d.). Research and Innovation.
- Leeds Teaching Hospitals NHS Trust. (n.d.). Research and Innovation.
- Leeds Teaching Hospitals NHS Trust. (2023). Our Research and Innovation Strategy 2023-2028.
- Leeds Teaching Hospitals NHS Trust. (n.d.). Innovation at LTHT.
- Innovation Pop Up. (n.d.). Welcome to the Innovation Pop Up.
- Leeds Teaching Hospitals NHS Trust. (2022). Digital IT Strategy 2022-2025.
- Care Quality Commission. (2023). Inspection report: Leeds General Infirmary.
- Care Quality Commission. (2023). Inspection report: St James's University Hospital.
- NHS Health Education England. (n.d.). Welcome to the Research Toolkit.
- Triantafyllidis, A., & Triantafyllidis, A. (2018). Human factors and user-centered design in digital health. Health Informatics: An Interprofessional Approach, 2, 293-316.
- Steen, M., Manschot, M., & De Koning, N. (2011). Benefits of co-design in service design projects. International journal of design, 5(2).
- NHS Health Education England. (n.d.). Research Toolkit Step 2: Involving People.
- Grant, K. A., McMahon, C., & Austin, M. P. (2008). Maternal anxiety during the transition to motherhood: A systematic review. Journal of affective disorders, 108(1-2), 101-111.
- Spitzer, R. L., Kroenke, K., Williams, J. B., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine, 166(10), 1092-1097.
- NICE. (2011). Generalised anxiety disorder and panic disorder in adults: management (CG113).
- Brooke, J. (1996). SUS-A quick and dirty usability scale. Usability evaluation in industry, 189(194), 4-7.
- Bangor, A., Kortum, P. T., & Miller, J. T. (2008). An empirical evaluation of the System Usability Scale. International Journal of Human-Computer Interaction, 24(6), 574-594.
- NHS Health Education England. (n.d.). Research Toolkit Step 3: Study Design.
- Craig, P., Dieppe, P., Macintyre, S., et al. (2008). Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ, 337.
- Bate, P., & Robert, G. (2006). Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Quality and Safety in Health Care, 15(5), 307-310.
- Creswell, J. W., & Clark, V. L. P. (2017). Designing and conducting mixed methods research. Sage publications.
- Lewis, J. R. (2018). The system usability scale: past, present, and future. International Journal of Human–Computer Interaction, 34(7), 577-590.
- Glasgow, R. E., Harden, S. M., Gaglio, B., et al. (2019). RE-AIM planning and evaluation framework: a systematic review of use and effectiveness. American journal of public health, 109(3), e1-e11.
- NIHR. (n.d.). Programme Grants for Applied Research (PGfAR).
- Kwan, B. M., McGinnes, H. L., Ory, M. G., et al. (2019). RE-AIM in the wild: a review of the RE-AIM framework in federally funded implementation and dissemination research. American journal of preventive medicine, 56(4), 609-619.
- Medicines and Healthcare products Regulatory Agency. (2023). Guidance: Software and AI as a Medical Device (SaMD).
- Health Research Authority. (n.d.). Medical devices.
- NHS Health Education England. (n.d.). Research Toolkit Step 4: Regulation and Governance.
- Health Research Authority. (n.d.). Integrated Research Application System (IRAS).
- Health Research Authority. (n.d.). Combined review.
- NHS Health Education England. (n.d.). Research Toolkit Step 5: Preparing the Application.
- Health Research Authority. (n.d.). HRA and HCRW Approval.
- Health Research Authority. (n.d.). UK Local Information Pack.
- NIHR. (n.d.). Schedule of Events Cost Attribution Template (SoECAT).
- NHS Health Research Authority. (2020). Organisation Information Document.
- NIHR. (2021). UK Standards for Public Involvement.
- INVOLVE. (2012). Briefing notes for researchers.
- NIHR. (n.d.). Patient and public involvement in research.
- NIHR. (2021). Payment and recognition for public involvement.
- NIHR. (2022). Challenge: Maternity Inequalities.
- Information Commissioner's Office. (n.d.). Guide to the UK GDPR.
- Information Commissioner's Office. (n.d.). What is special category data?.
- Data Protection Act 2018.
- Information Commissioner's Office. (n.d.). Lawful basis for processing.
- Health Research Authority. (n.d.). GDPR guidance for researchers.
- Health Research Authority. (n.d.). Appropriate policy document.
- Information Commissioner's Office. (n.d.). Consent.
- Health Research Authority. (n.d.). Informed consent.
- Information Commissioner's Office. (n.d.). Data protection impact assessments.
- Information Commissioner's Office. (n.d.). Right to be informed.
- Information Commissioner's Office. (n.d.). Privacy notices, transparency and control.
- UK Data Service. (n.d.). Anonymisation.
- Digital Curation Centre. (n.d.). DMPonline.
- UKRI. (n.d.). Research data management and sharing.
- NHS Health Education England. (n.d.). Research Toolkit Step 8: Data Management and Analysis.
- NHS Health Education England. (n.d.). Research Toolkit Step 7: Managing the Project.
- NIHR. (n.d.). Tips for writing a strong grant application.
- NHS Health Education England. (n.d.). Research Toolkit Step 9: Dissemination.
- NIHR. (n.d.). How to disseminate your research.
- UKRI. (n.d.). Open access policy.
- Medical Research Council. (n.d.). Developmental Pathway Funding Scheme (DPFS).
- NIHR. (n.d.). Invention for Innovation (i4i).
- NHS Health Education England. (n.d.). Research Toolkit Step 10: Getting Your Research Funded.
- NIHR. (n.d.). i4i Connect.
- NIHR. (2022). Programme Grants for Applied Research: Applicant Guidance.
- NIHR. (n.d.). i4i Product Development Awards (PDA).
- Wellcome Trust. (n.d.). Mental Health.
- Wellcome Trust. (n.d.). Funding for mental health research.